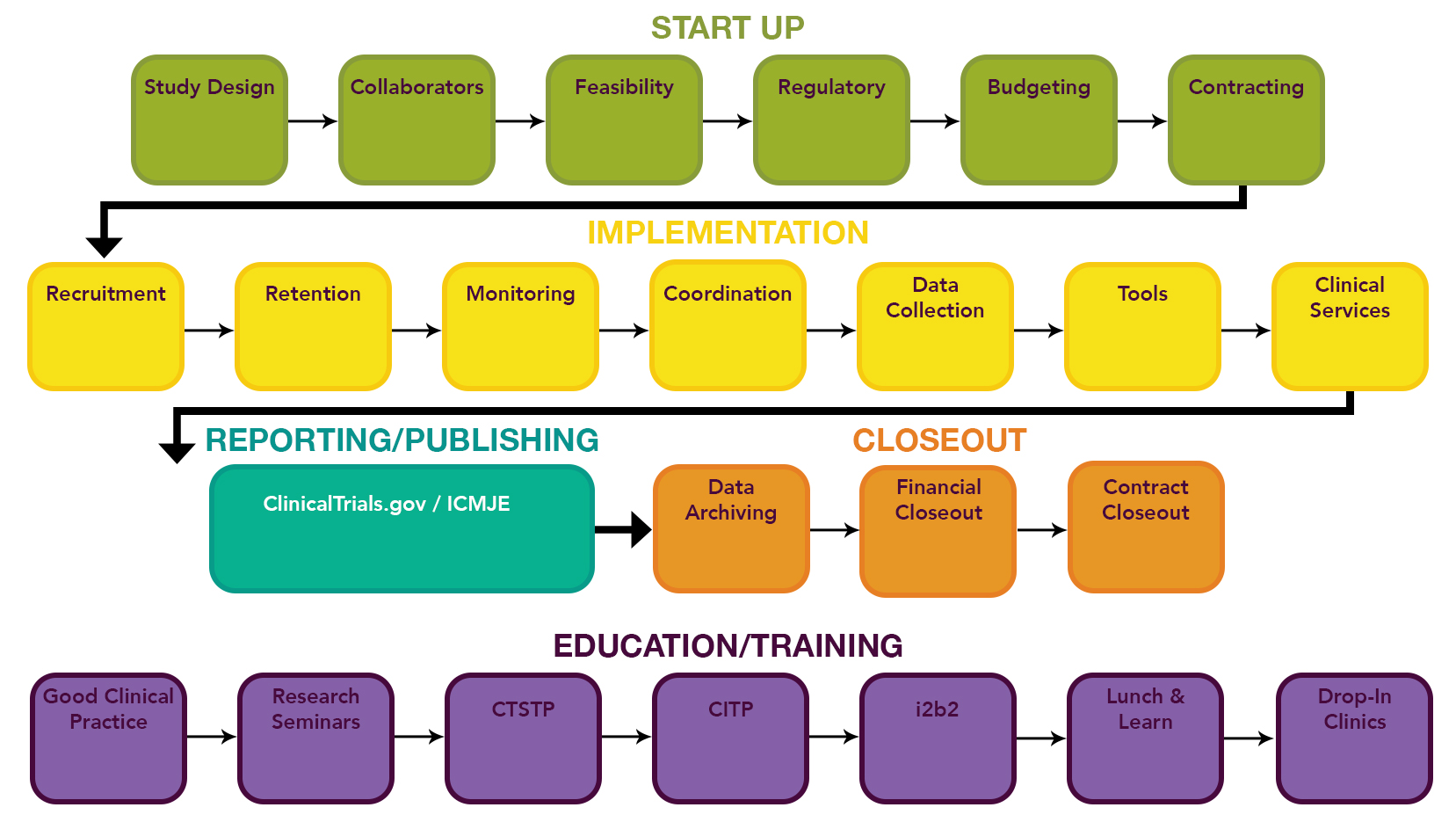
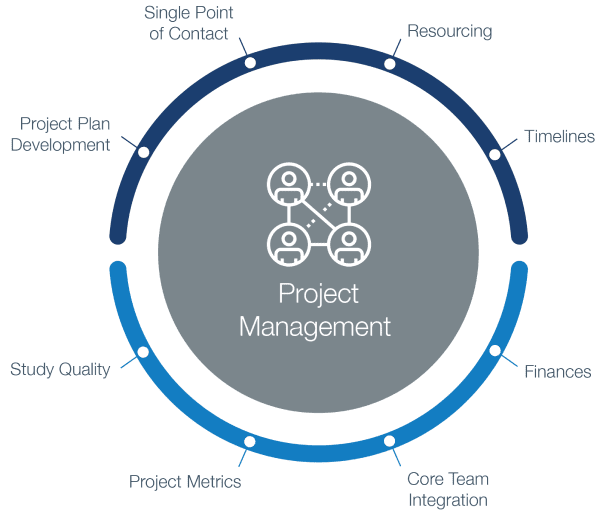
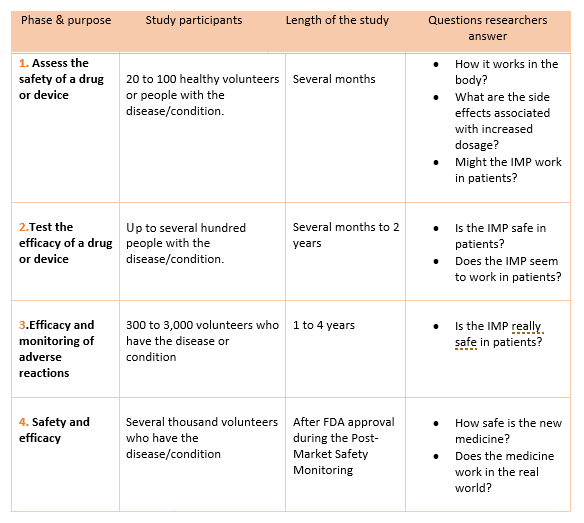
Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study - The Lancet
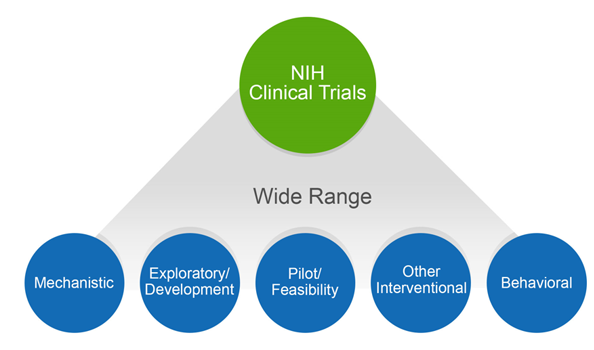
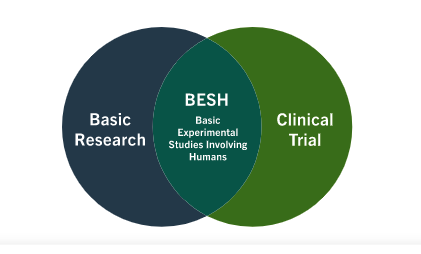
Guidelines for clinical trial protocols for interventions involving artificial intelligence: the SPIRIT-AI extension | Nature Medicine

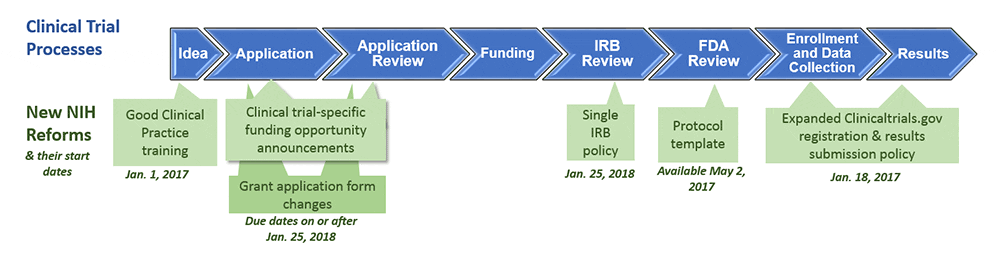
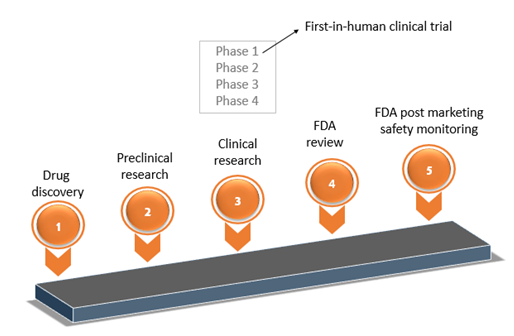
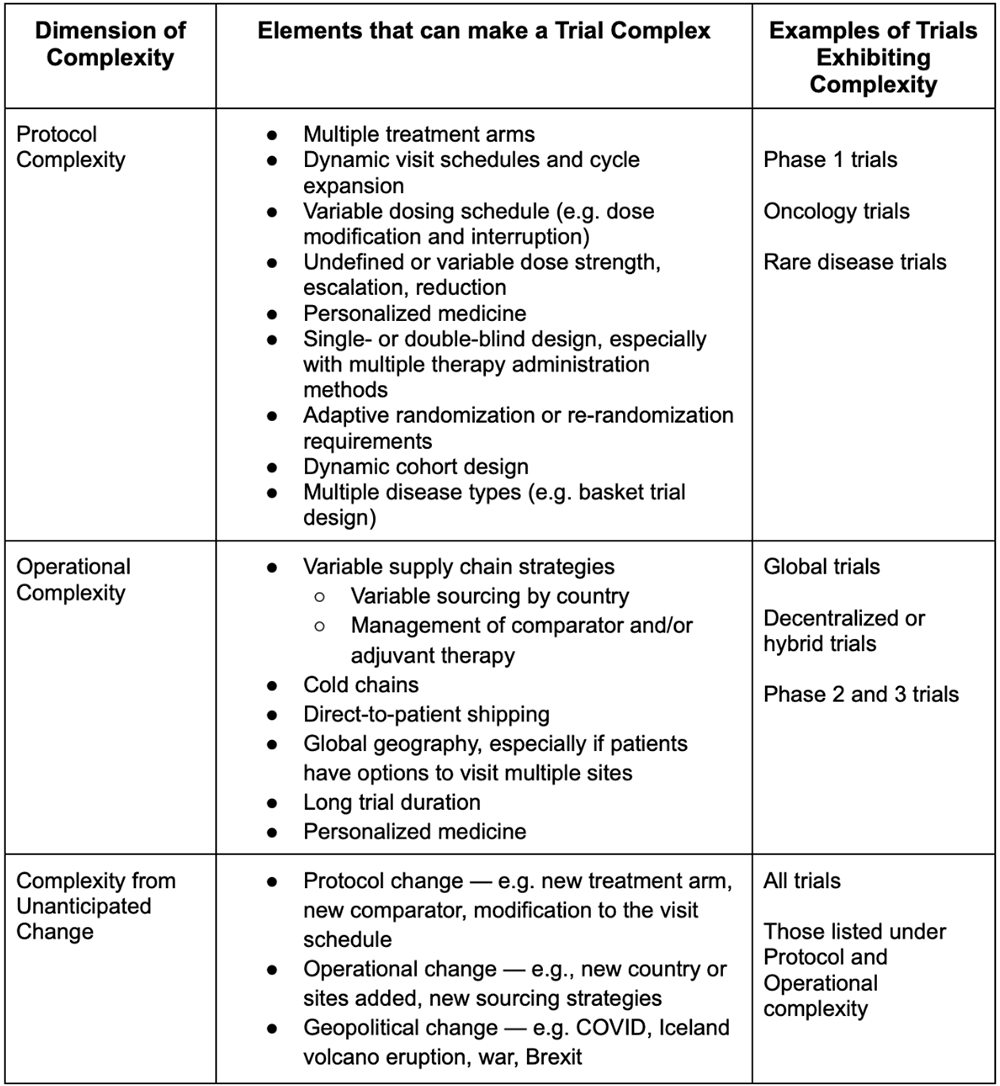
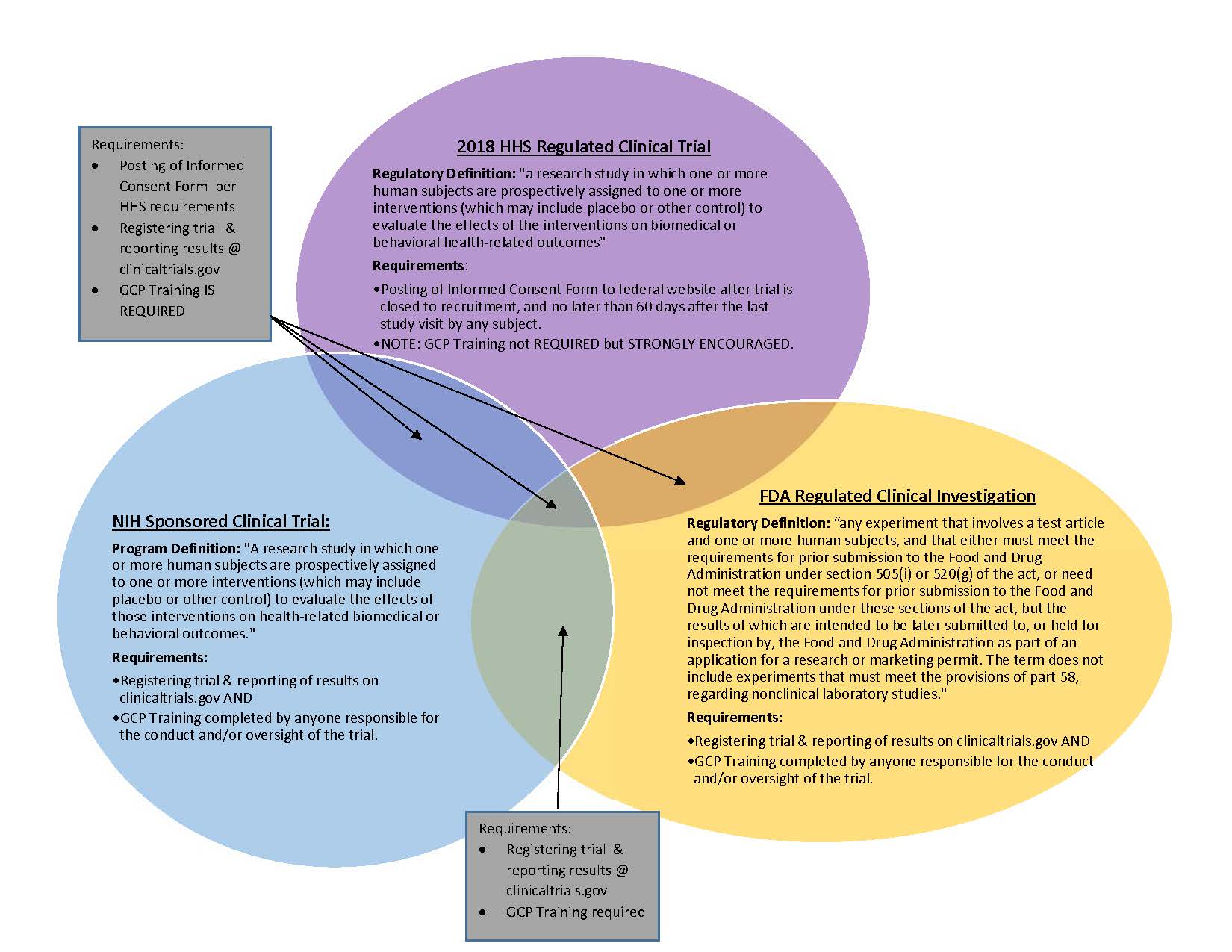
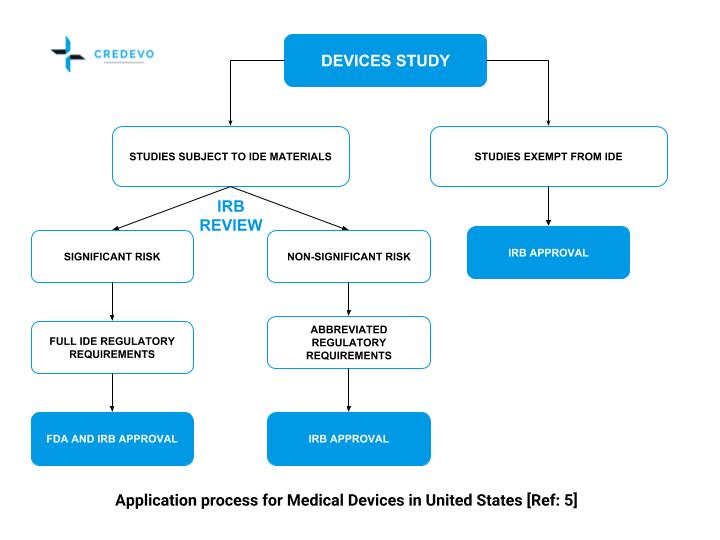
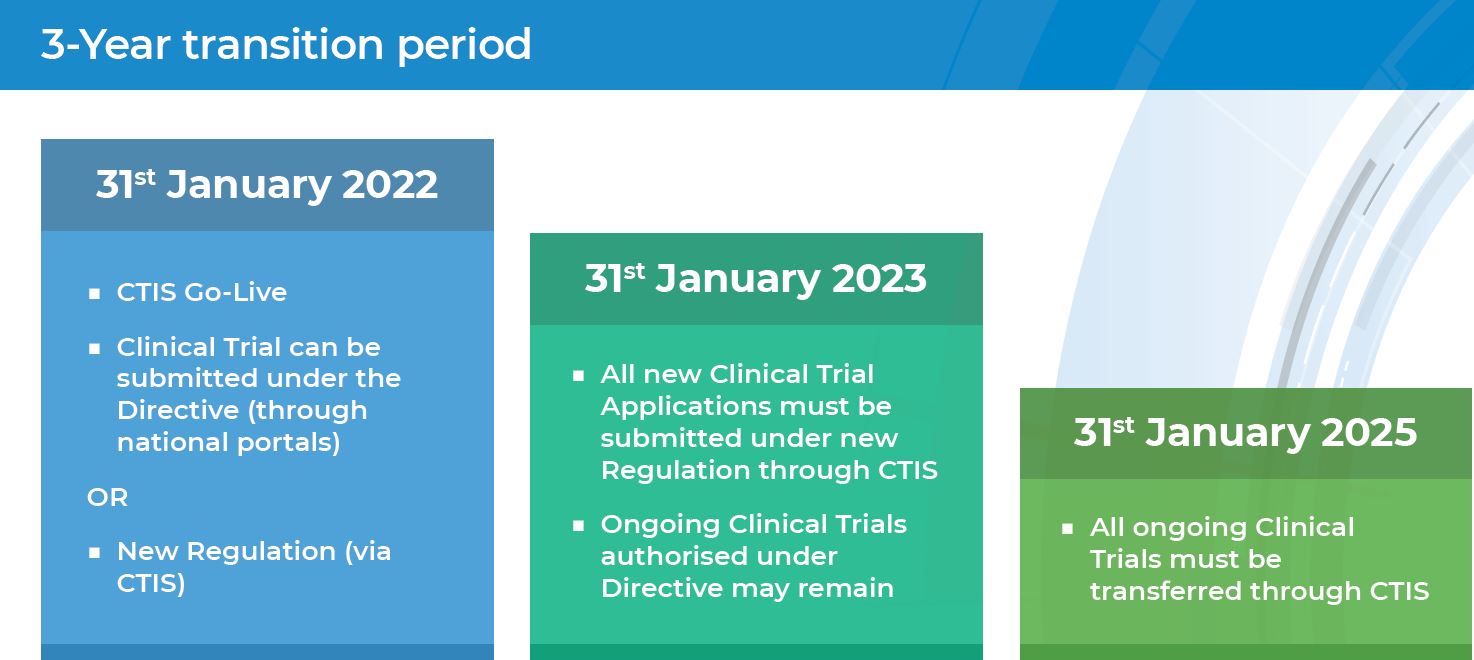
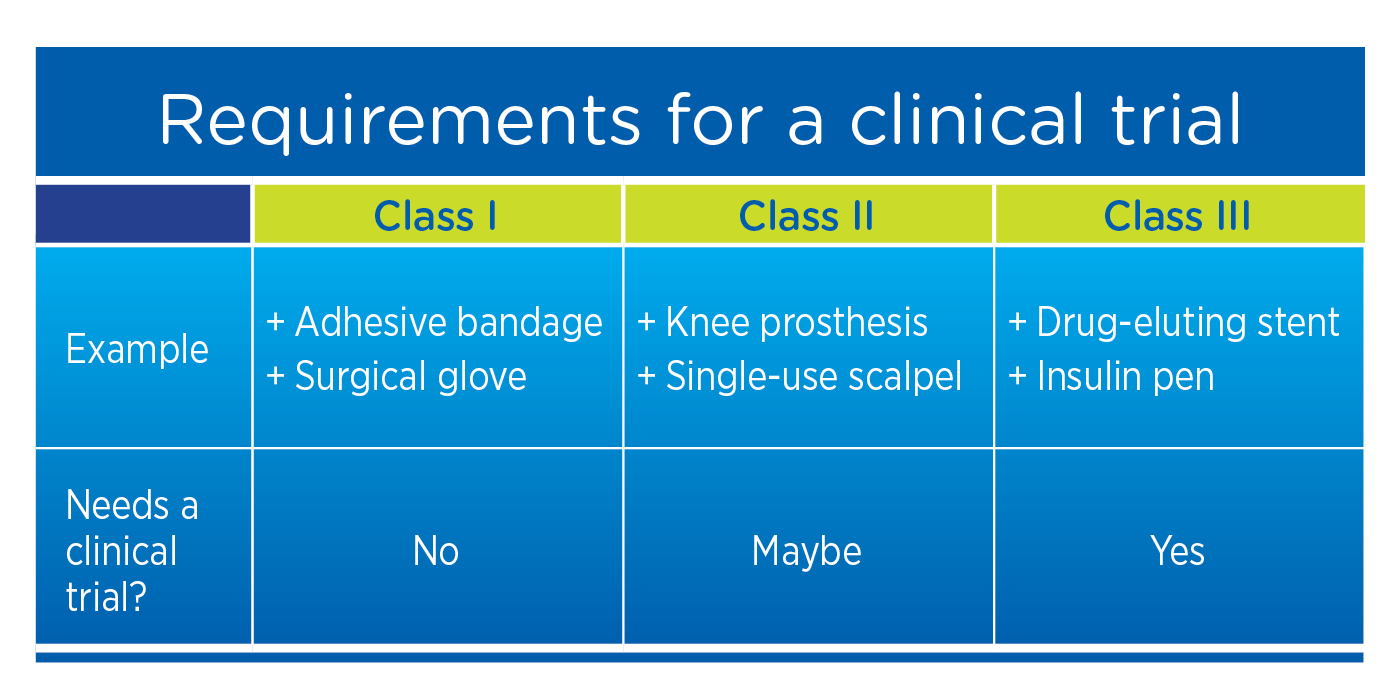
ADVANCED CLINICAL TRIALS THE CLINICAL TRIAL PROCESS: IMPENDING CHANGES IN THE REGULATORY FRAMEWORK - ADVANCED CLINICAL TRIALS