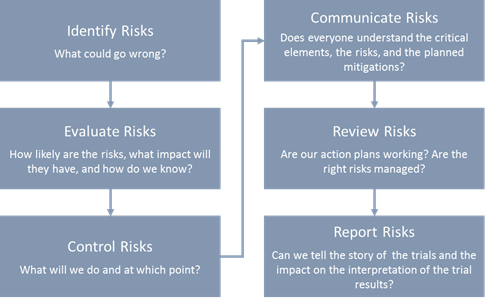
GCP Finding - Good Clinical Practice (GCP) FREE online training is based on the ICH E6 (R2) Guideline. The training contains presentations that will allow participants to enjoy the learning process and

The Impact of ICH GCP E6 Guideline R2 Revisions on Sponsors, Sites, Contract Research Organizations and Vendors | Pharmaceutical Outsourcing - The Journal of Pharmaceutical & Biopharmaceutical Contract Services

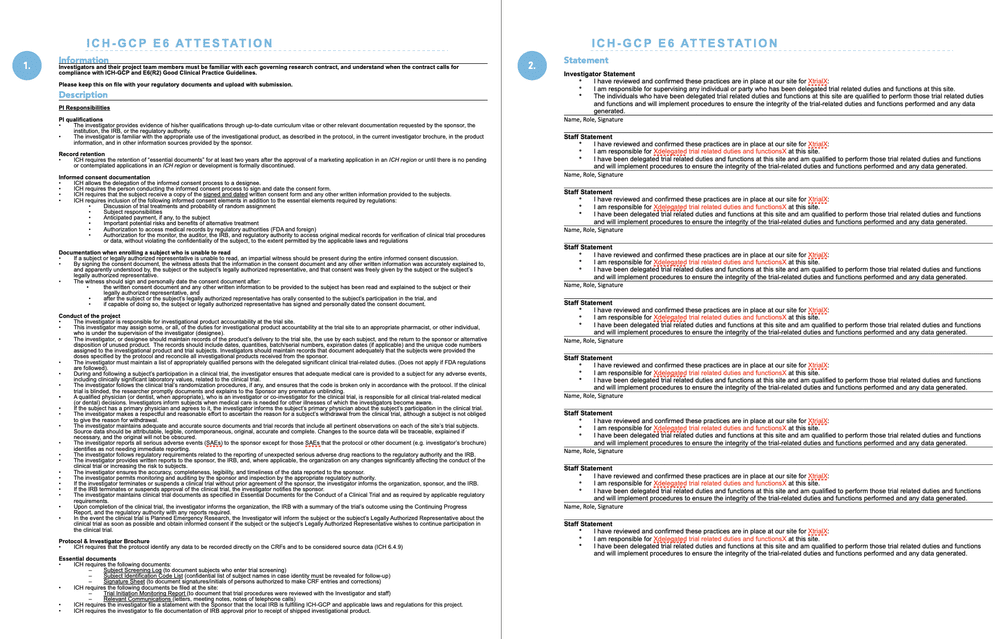
ICH GCP - 8. Essential documents for the conduct of a clinical trial: ICH E6 (R2) Good clinical practice

E6(R2) Good Clinical Practice Integrated Addendum to ICH E6(R1) - E6(R2) Good Clinical Practice: - Studocu

The Impact of ICH GCP E6 Guideline R2 Revisions on Sponsors, Sites, Contract Research Organizations and Vendors | Pharmaceutical Outsourcing - The Journal of Pharmaceutical & Biopharmaceutical Contract Services
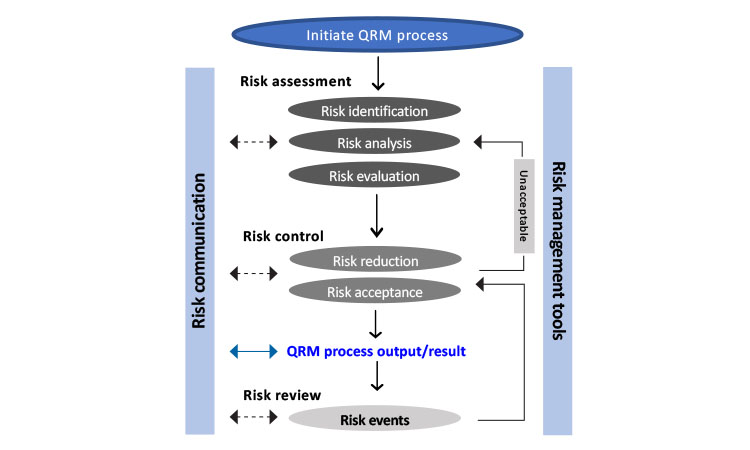
Clinical Trial Management Adaptation to ICH E6 (R2): Good Clinical Practice | Pharmaceutical Engineering






.jpg)