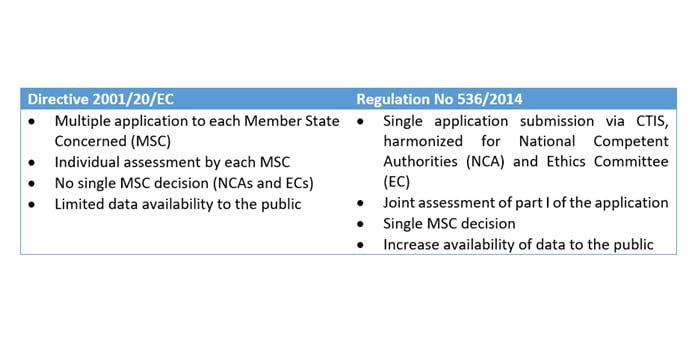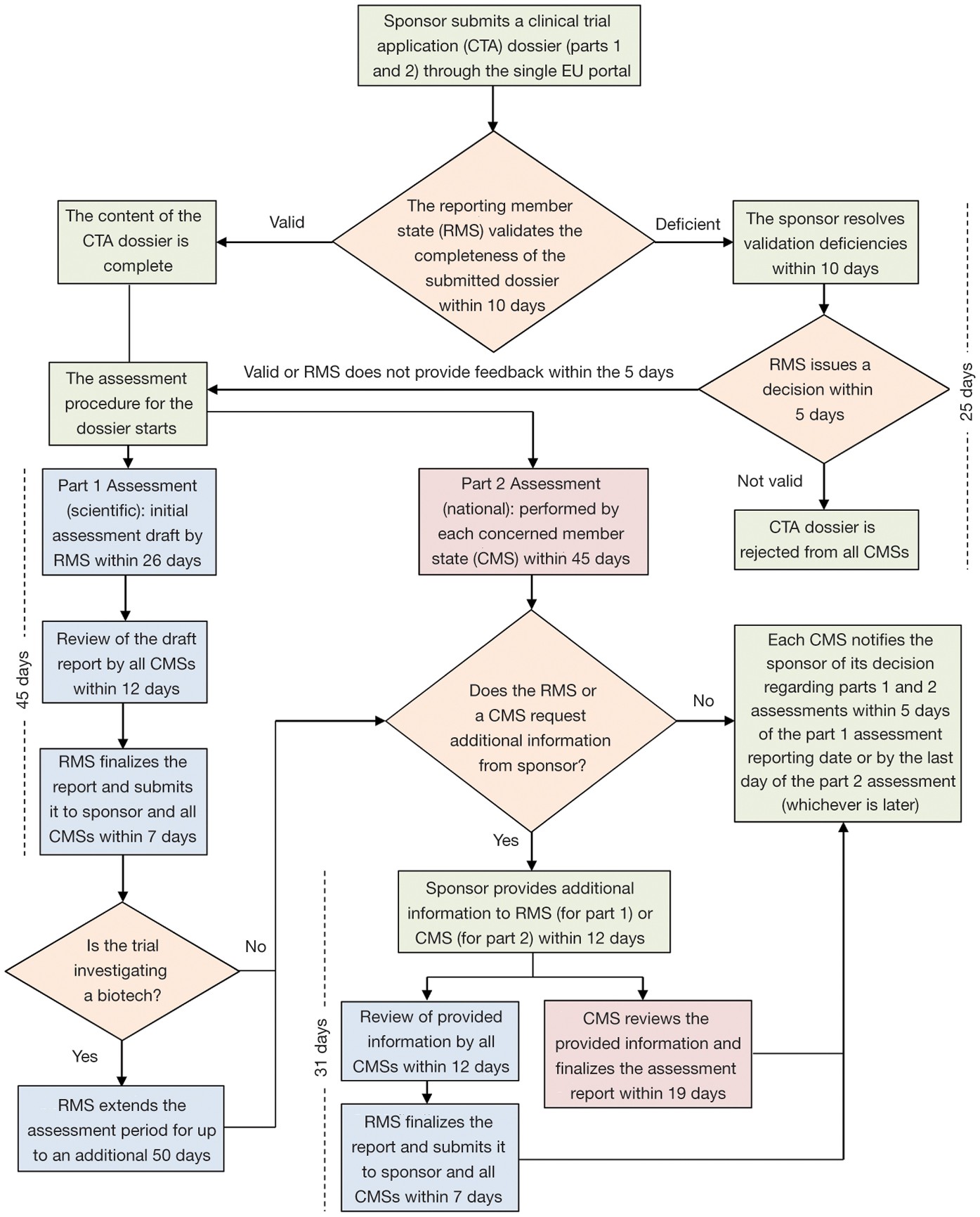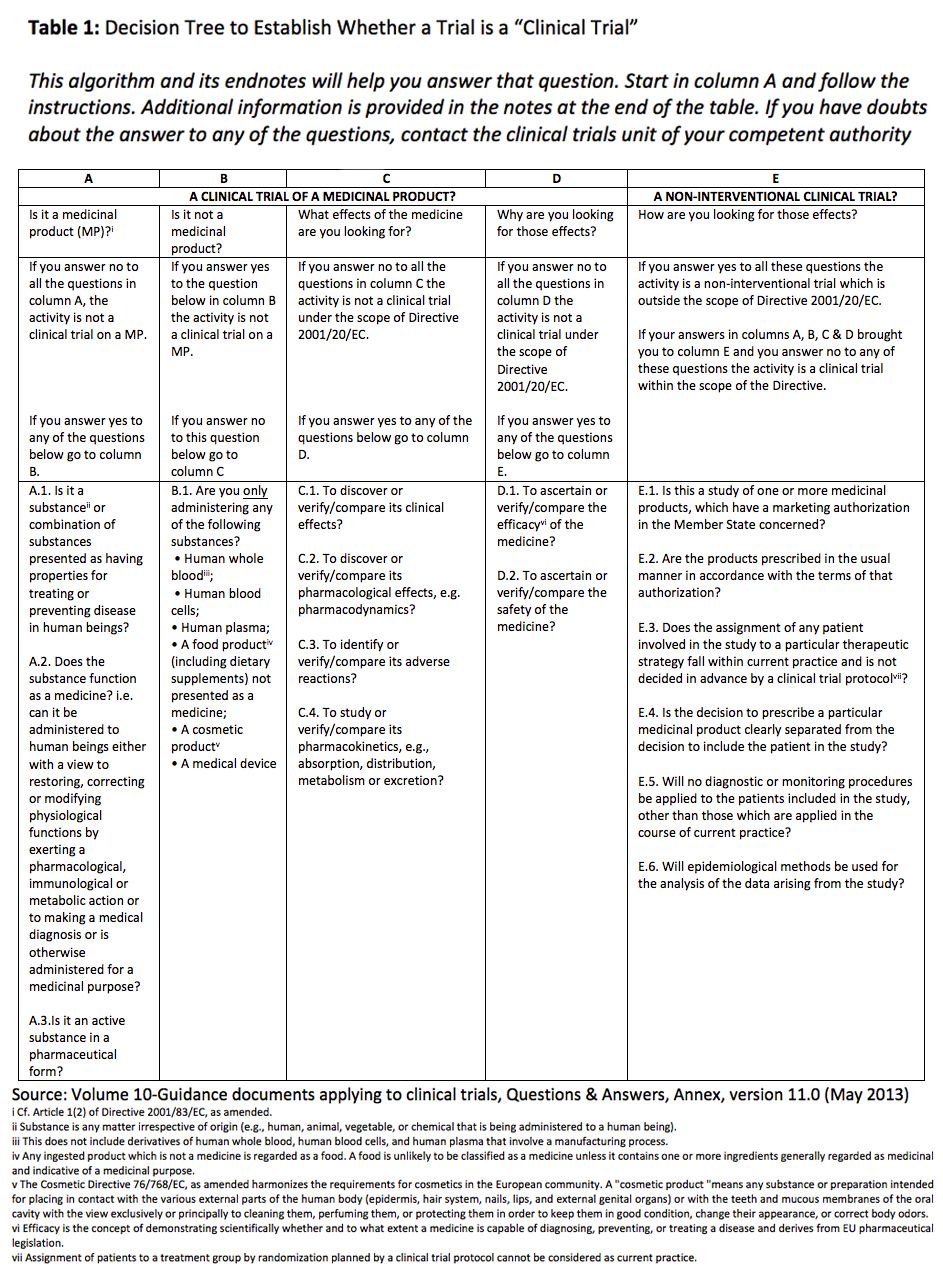
Interventional vs. Non-interventional Study Classification in the EU: Considerations on the Impact of Direct-to-Patient Contacts

Timeline impact assessment and Revision of Directive 2001/20/EC (see... | Download Scientific Diagram

Directive 2001/20/EC : Clinical trials on medicinal products for human use - Free PDF download | M A N O X B L O G

European Clinical Trial Directive (Directive 2001/20/EC) dr. Cees Smit (NPCF/EGAN) EPF Annual Meeting May 19, Brussels. - ppt download
PDF) Typical investigational medicinal products follow relatively uniform regulations in 10 European Clinical Research Infrastructures Network (ECRIN) countries

White Paper: Pharmaceutical Industry Challenges Facing Clinical Trial Disclosure and Transparency - TrialAssure

Will the EU Clinical Trials Regulation Support the Innovative Industry in Bringing New Medicines Faster to Patients? | SpringerLink

Exploring the Impact of the New European Directive on the Pharmaceutical Industry - Clinical Trials Arena
EUROPEAN COMMISSION Brussels, 11/04/2012 sanco.ddg1.d.6(2012)501417 VOLUME 10 - G Date of discussion of draft by the ad-hoc gro

When innovation outpaces regulations: The legal challenges for direct‐to‐patient supply of investigational medicinal products - Malone - 2022 - British Journal of Clinical Pharmacology - Wiley Online Library

GCP and Quality in “Regulation (EU) 536/2014 on clinical trials on medicinal products for human use and repealing Directive 2001/20/EU” - ScienceDirect

EORTC EU Clinical Trials Directives Organisation and Implementation of Cancer Clinical Trials Anastassia Negrouk EORTC Regulatory Affairs Manager Intergroup. - ppt download