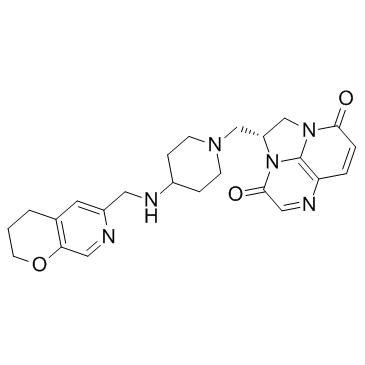
Mechanistic and Structural Basis for the Actions of the Antibacterial Gepotidacin against Staphylococcus aureus Gyrase | ACS Infectious Diseases
First Novel Antibiotic Since 20 Years, Gepotidacin Debuts To Treat Drug Resistance Urinary Tract Infections (UTIs) Amidst Growing Incidences! - Thailand Medical News
Antibiotics | Free Full-Text | Antibacterial Activity of the Novel Drug Gepotidacin against Stenotrophomonas maltophilia—An In Vitro and In Vivo Study

Pharmacokinetics, safety, and tolerability of gepotidacin administered as single or repeat ascending doses, in healthy adults and elderly subjects - Tiffany - 2022 - Clinical and Translational Science - Wiley Online Library

Dose Selection for Phase III Clinical Evaluation of Gepotidacin (GSK2140944) in the Treatment of Uncomplicated Urinary Tract Infections | Antimicrobial Agents and Chemotherapy

Dose Selection for Phase 3 Studies Evaluating Gepotidacin (GSK2140944) in the Treatment of Uncomplicated Urinary Tract Infections | GSK
Phase 2a Pharmacokinetic, Safety, and Exploratory Efficacy Evaluation of Oral Gepotidacin (GSK2140944) in Female Participants wi

Dose Selection for Phase 3 Studies Evaluating Gepotidacin (GSK2140944) in the Treatment of Uncomplicated Urinary Tract Infections | GSK
Gepotidacin for the Treatment of Uncomplicated Urogenital Gonorrhea: A Phase 2, Randomized, Dose- Ranging, Single-Oral Dose Eva

Dose Selection for a Phase 3 Study Evaluating Gepotidacin (GSK2140944) in the Treatment of Gonorrhoea | GSK

Overview of microbiome dynamics during gepotidacin Phase 2a clinical... | Download Scientific Diagram

Dose Selection for Phase III Clinical Evaluation of Gepotidacin (GSK2140944) in the Treatment of Uncomplicated Urinary Tract Infections | Antimicrobial Agents and Chemotherapy

Pharmacokinetics of Oral Formulations of Gepotidacin (GSK2140944), a Triazaacenaphthylene Bacterial Type II Topoisomerase Inhibitor, in Healthy Adult and Adolescent Participants | Antimicrobial Agents and Chemotherapy
Design of Two Phase III, Randomized, Multicenter Studies Comparing Gepotidacin with Nitrofurantoin for the Treatment of Uncomplicated Urinary Tract Infection in Female Participants | SpringerLink

Mechanistic and Structural Basis for the Actions of the Antibacterial Gepotidacin against Staphylococcus aureus Gyrase | ACS Infectious Diseases
Gepotidacin for the Treatment of Uncomplicated Urogenital Gonorrhea: A Phase 2, Randomized, Dose- Ranging, Single-Oral Dose Eva
Antibiotics | Free Full-Text | Pharmaceutical Approaches on Antimicrobial Resistance: Prospects and Challenges